After years of building up their drug production in countries with lower operating costs and lower taxes, pharma companies supplying the US market could soon face an urgent need to rethink where they manufacture what they sell.
The Trump administration and Congress are considering an array of measures that have far-reaching implications for pharma leaders. Potential changes to US tax policies include the following:
- Border adjustability (taxing imports and instituting tax-free exports)
- Reduction of the corporate income tax rate from its current 35% to as low as 15%
- Adoption of territorial taxation (imposing no taxes on new foreign earnings)
- Taxation of prior foreign-held earnings, including optional and mandatory taxes
- Alterations in US trade policy, including tariffs
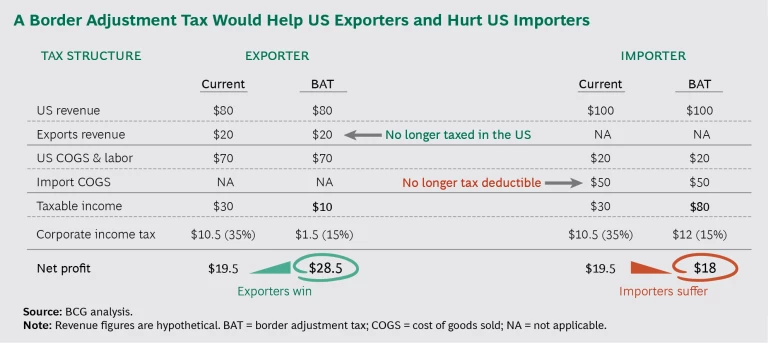
The impact of just the import portion of a border adjustment tax (BAT) would be quite significant. Pharma companies supplying the US, particularly those focused on small molecules, obtain 80% of their active pharmaceutical ingredients and 40% of their finished drug products from overseas manufacturing facilities. An import tax could substantially decrease pharma profits.
Enactment of any of these proposals could bring fundamental changes to the global complexities of pharma supply chains and product networks. Companies across the industry would feel the impact of the changes differently, creating both challenges and opportunities for all players. Companies that understand the implications of tax policy shifts will be better prepared to act if economic considerations ultimately favor relocating production.
Existing Policy Pushes Manufacturing Offshore
The current US corporate tax (35%) is well above the average tax rate among developed nations (the OECD average is 25%). Current US tax policies, in combination with attractive policies elsewhere, have encouraged global pharma companies to maximize their profits by placing manufacturing operations to other regions.
Developed nations such as Ireland, Singapore, and Belgium offer attractive combinations of lower taxes, a highly skilled workforce, opportunities for academic collaboration, IP protection, and streamlined regulation. Developing countries such as China and India offer labor rates that may be as low as one-tenth those in the US, along with significantly lower construction, energy, and other costs. As a result, many pharma companies have located not just their manufacturing, but also their IP and—in some cases—their corporate headquarters overseas.
As of this writing, the Trump administration has offered few specifics with regard to its planned overhaul of the federal tax system. One of its stated goals, however, is to close trade deficits and shift manufacturing jobs in all industries back to the US. Pharma companies must understand the implications for their industry of whatever program emerges in the next year and must anticipate how the new policies could affect their decisions about where to manufacture and sell their products.
Five Key Implications of Proposed Tax Policy Changes
In light of the Trump administration’s intention to reform the tax code, pharma leaders should begin evaluating their supply networks’ exposure, in the event that these policies become law. The potential tax policy changes have at least five significant implications:
- Denying cost deductions for imports would be tantamount to imposing an import tax. This policy change would put companies that import drug inputs and finished products into the US in a less competitive position than they currently occupy. Under certain conditions, the new policies might serve as an incentive for companies to reshore manufacturing to the US (or re-source inputs now procured from overseas).
- Introducing tax-free exports would strengthen the competitiveness of US net exporters. This policy change would give US-produced goods an advantage in foreign markets and might entice manufacturers that seek low taxes for goods consumed outside the US to reshore production to the US. Tax-free exports might be especially alluring in connection with intangible assets such as IP, which do not have physical transport implications. This advantage would remain in effect unless the US dollar appreciated to the point at which the foreign exchange rate matched the tax change, or until other countries mounted a reciprocal response to a new US trade policy.
- The US is currently one of the few nations to tax overseas, as well as domestic, earnings. The proposed territorial system would no longer tax foreign profits, which would affect how pharma companies treat foreign earnings. The proposed transition tax for any prior earnings currently held overseas might prompt companies to consider options for handling overseas cash they now hold. Such actions might include accelerating planned overseas investment decisions (if they still made sense, given other tax changes). In addition, US companies might choose to accelerate asset acquisitions under the current higher rates of depreciation deduction. They might also choose to delay disposing of assets until lower rates for gains were in place. In deciding where to hold profits, companies would also have to consider likely changes in currency value.
- If the tax treatment of US imports were to change, pharma companies would need to rethink their internal pricing structures and strategies. This might lead to the decoupling of IP and manufacturing, since companies would no longer have an incentive to co-locate the two as part of their internal pricing strategies. Separating intangible assets from manufacturing might lead companies to restructure their supply chain footprint. It would also create strong incentives to reshore IP to the US to reduce global tax exposure and to take advantage of US export incentives. The separation might even cause companies to dramatically increase their outsourcing as the need for physical assets in low-tax regions diminished. New pricing strategies that rely on IP or manufacturing location might become attractive. But companies would need to be cautious, given tax authorities’ interest in any such pricing shifts.
- US enactment of a border adjustment tax would likely trigger a significant international response. If other countries moved to counteract the imbalance created by the BAT, the benefit to US exporting might disappear. A shift toward greater economic protectionism across the globe might encourage biopharma companies to create relatively fragmented, localized manufacturing to offset increased costs associated with moving product across trade borders. The reduction in scale resulting from fragmentation would likely increase unit costs, driving the need for improvements in efficiency (such as accelerated movement toward Industry 4.0).
The Impact of Tax Policy Changes Will Vary Across the Industry
With so many major policy proposals in play, predicting winning and losing sectors within the pharma industry is difficult. (See the exhibit.) In a BAT-imposition-pluscorporate- tax- reduction scenario, we might see the following effects on hypothetical examples of four types of companies.
US-based biologics companies serving a global market would be big winners. Companies of this type have the most to gain from new tax policies, particularly if their businesses do not import inputs from overseas. These companies would face a lower corporate tax rate, and their export tax-free profits from overseas sales would reduce their overall tax exposure. They would also enjoy a pricing advantage in international markets over competitors that cannot discount on the basis of tax-free export status. Companies for which US production is already attractive in the current environment would likely find that the revised tax policies further increased the benefit of their current supply chain.
Small US-based OTC producers serving the US domestic market would be modest winners. In comparison with foreign competitors, these companies stand to benefit somewhat from a reduced corporate tax and from new tax advantages of manufacturing in the US. The benefit that these companies would realize depends on whether and at what stage they brought in inputs from abroad. For example, small US-based producers would benefit more than overseas competitors if they imported raw chemicals rather than APIs. Companies should identify the stages or inputs (if any) to their supply chain that rely on foreign inputs. They could then assess the net value of using those inputs, in light of the increased tax burden, as opposed to using alternative domestic US sourcing options.
Foreign-based generics companies selling to the US market would be worse off. If an import tax were enacted, offshore companies would face an immediate cost hike for product they imported into the US. Tax-free exportation would not benefit them, and neither would lower US corporate taxes or tax benefits for US manufacturing. If their margins were already low due to competitive market dynamics, they might find it difficult to compete profitably with domestic US rivals. In organizing their supply chains, these companies might need to focus on reducing overall costs to stay competitive, given the new import penalties. Or they might need to consider shifting value-add portions of their supply chain to the US.
Foreign-based innovative companies with mixed assets and a global market might ultimately be worse off. A foreign-based company with assets in the US and other markets would encounter a blend of the issues highlighted above. Although most large pharma companies locate some of their production in US facilities, many of them import parts of their supply chain into the US. These imports often take place during the earlier stages of production. A rise in internal pricing of higher-value API materials might significantly increase such a company’s tax exposure. The right balance between domestic and offshore production stages would depend on how much of the US production was subsequently exported.
How to Prepare for Potential Tax Policy Changes
Implementation of any of the tax policies currently under consideration is far from certain. The Trump administration’s difficulty (thus far) in shepherding changes to the US health care system through Congress serves as a warning. It may not be easy for the administration to convince Congress to adopt policies that retool the entire corporate tax structure. Nevertheless, biopharma companies should be prepared to adapt—and in some cases they may need to be ready to move quickly.
To prepare for potential changes to US tax policy, biopharma companies should take the following steps:
- Assess the degree of exposure. Companies should take steps now to review the implications to their business of any new tax policy or combination of policies under consideration. With supply chains that stretch across continents, and with complex profit-maximization strategies, pharma companies may find the task of assessing their exposure quite challenging. Furthermore, the degree of exposure is likely to vary depending on the products they manufacture. As a result, though the considerations will be the same from one company to the next, the weight of each will probably differ. For example, many companies’ supply chains for producing large-molecule products already include US production, while production of supply-chain components for small-molecule products are more likely to be imported to the US.
- Consider whether and to what degree shifting production to the US would be advantageous. If the US government enacts a modest reduction in the corporate tax (an idea that has strong support), it might be more advantageous for companies to build new production facilities in the US than in low-tax regions. If an import tax is adopted as part of a BAT, reshoring production to the US may become even more attractive. Enactment of a tax-repatriation holiday would give companies access to additional capital needed to move their production facilities. Again, this analysis will vary depending on the types of products a particular company produces. The higher capital expenditures commonly required for large-molecule production facilities may create a higher barrier to shifting their production to the US than to hindering reshoring of small-molecule production. With everything else being equal, companies are likely to have a stronger incentive to shift production of lower-margin products to the US. This is because the incremental tax burden for these lower-margin products will have a larger impact on profitability (on a percentage basis) than the corresponding burden for higher-margin products.
- Explore options to increase US production, including the use of local CMOs. Companies interested in reshoring to the US have a number of production options. The most favorable of these would be to use their existing US manufacturing capacity. If sufficient capacity is unavailable, companies may consider expanding their own plants, purchasing capacity from other companies, or outsourcing to US-based CMOs.
- Investigate potential upstream impacts. Changes in US tax policy may also affect pharma suppliers. Suppliers that anticipate a significant impact from such changes may be inclined to pass the cost increases on to their pharma clients. Pharma companies would be well served to understand the locales of their suppliers—and of their suppliers’ suppliers. Such knowledge could enable pharma companies to anticipate potential impacts of policy change on their suppliers, as well as to explore developing relationships with US-based suppliers.
Effecting any of these supply chain changes is a nontrivial exercise in time (likely a multiyear effort, given technical and regulatory timelines), cost (one multinational pharma has estimated a capital investment of over $1 billion to reshore production for US sales), and complexity. That said, if the tax and trade policies discussed here are enacted, the benefits of adopting these supply chain changes—or the cost of not doing so—may justify the investment for many pharma companies.
The lack of consensus on tax policy direction within the government and across industries clouds the prospects for comprehensive corporate tax reform. As a result, companies are not likely to make any decisions about shifting supply chains to the US yet, and they may choose to delay other potential network changes. Nevertheless, pharma companies should monitor and participate in conversations regarding policy reform, and should be ready to respond quickly as legislation moves forward.