Data and analytics are now widely used in business to solve a range of problems. In health care, the stakes are arguably much higher, but the tools are just as applicable. Increasingly, large data sets and powerful analytics can be used to cut through the fog that clouds decision making, guiding physicians and clinicians toward the best diagnostic tests and care pathways—ultimately leading to better outcomes.
To show the power of this approach, BCG recently partnered with AdvaMedDx, an industry association of medical diagnostics companies, to examine the barriers to adoption for potentially lifesaving diagnostic tests. The team agreed to use the adoption of epidermal growth factor receptor (EGFR) diagnostics among patients with metastatic nonsmall cell lung cancer (NSCLC) as the test case, and BCG evaluated a broad sampling of US patients.
The results showed that roughly 30% of patients presenting with this disease from 2011 through 2017 did not receive this critical and clinically indicated diagnostic test. Extrapolating the results to the US population, that translates into 43,000 metastatic NSCLC patients. As a result, some patients may have experienced delays in starting chemotherapy treatment or received an inappropriate treatment, possibly resulting in faster disease progression and death. Why did some patients receive the diagnostic while others did not? Surprisingly, the biggest factor was their oncologists, who have wide discretion with regard to ordering the test.
These findings are striking, and they underscore the clarity that proper analysis across large data sets can create. By using these tools, physicians, health systems, biopharma companies, and other stakeholders can better assess the quality of care a patient population receives, identify the root cause of variations in care, and ultimately help patients live longer, healthier lives.
The clarity created by the proper analysis of large data sets is striking.
Applying Data and Analytics to Diagnostics
Innovation in clinical diagnostics has brought significant value to health care and led to improvements in the oncology patient’s journey. Physicians have new ways to predict which patients have a high risk of developing a disease, better assays to diagnose patients, improved test panels to help personalize therapies, and more relevant approaches to monitoring chronic diseases. But patients do not reap the benefits of these new innovations unless payers, health systems, and clinicians adopt the new protocols in their clinical practices.
According to the guidelines of the National Comprehensive Cancer Network, all patients with advanced NSCLC should receive an EGFR test to determine if certain chemotherapy agents—namely, tyrosine kinase inhibitors (TKIs)—should be used. In patients with a relevant mutation of the EGFR gene, TKIs can substantially improve the overall survival rate; the benefit outweighs the risks. Conversely, in patients without an EGFR mutation, TKIs have no therapeutic benefit; they expose patients to toxicities but don’t improve their chances of living.
Thus, it is critical to test patients with this disease for EGFR mutations before deciding on a chemotherapy regimen.
Using a data set of deidentified electronic health records from the health care services company Optum®, we examined 5,687 records of individuals in the US who were diagnosed with metastatic NSCLC from 2011 through 2017. We analyzed the records to determine the following:
- The use of EGFR testing in patients with this disease
- The disparities in testing and the potential drivers of those gaps
- The impact of oncologists’ individual testing rates on the likelihood of patient survival
- The relationship between EGFR testing and TKI treatment
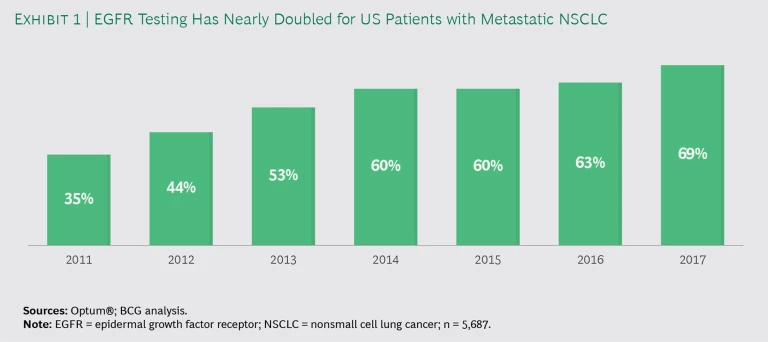
Oncologists are increasingly testing patients. From 2011 through 2017, the percentage of metastatic NSCLC patients who received a test to evaluate EGFR mutations nearly doubled, from 35% to 69%. (See Exhibit 1.) That is notable progress. Still, the failure to test approximately 30% of the patients for EGFR mutations means that some 1,700 patients in this study may have experienced delays in starting chemotherapy or received an inappropriate treatment, possibly resulting in faster disease progression and death.
The choice of an oncologist is the biggest factor behind use disparities. An evaluation of patient and physician factors that were associated with the likelihood of EGFR testing showed that men were 40% less likely to receive the test than women. Perhaps not surprisingly, patients with no insurance were 50% less likely to have an EGFR test than insured patients. However, the largest driver of use disparities was the managing oncologist, whose individual preference for ordering an indicated medical diagnostic often determines whether a patient receives that test.
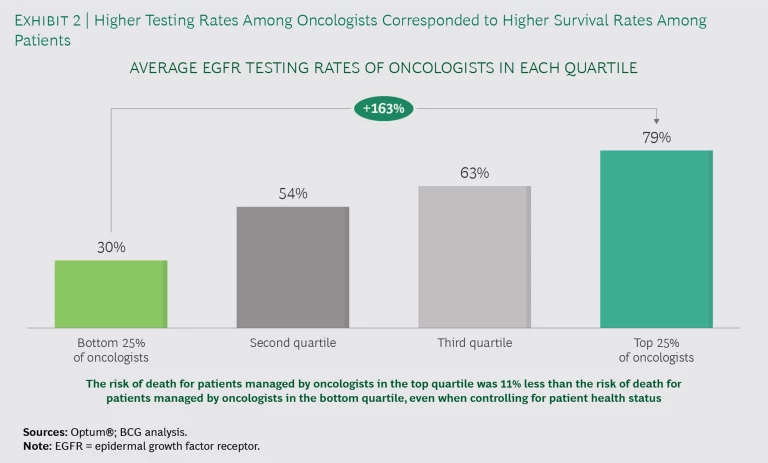
Variations in testing rates correspond to changes in survival rates. We segmented oncologists into quartiles on the basis of their EGFR testing rates. (See Exhibit 2.) The oncologists in the top quartile were more than twice as likely to order the EGFR test as those in the bottom quartile. Even after adjusting for patient factors, patients treated by top-quartile oncologists saw an 11% decrease in mortality rate, compared with patients treated by bottom-quartile oncologists.
Some patients received unnecessary chemotherapy, while others who needed treatment did not get it. Our analysis of the sample showed that 16% of the patients received TKI therapies (such as Tarceva, Iressa, and Tagrisso) without EGFR testing. Of those patients, only 30% were likely to have a EGFR gene mutation and therefore benefit from these drugs. The remaining 70% most likely received these treatments unnecessarily, putting them at risk from side effects—including immune system suppression, liver toxicity, and gastrointestinal problems—without any chance of benefiting. A secondary but not insubstantial issue is cost; these medications typically run about $10,000 per month.
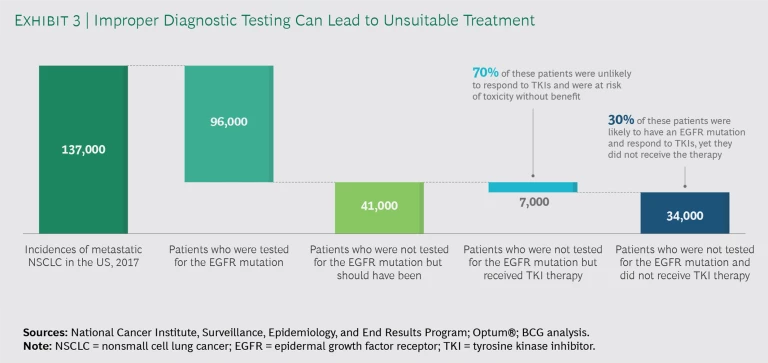
We extrapolated our findings to estimate the impact on the broader US population. (See Exhibit 3.) In 2017, there were about 137,000 incidences of advanced NSCLC in the US, and approximately 30%, or 41,000 patients, were not tested for EGFR mutations. Of those, roughly 7,000 were placed on TKI therapy, which exposed them to iatrogenic harm without the potential benefit. Additionally, by not testing some 34,000 patients with this disease, more than 10,000 life years were lost.
Translating Insights into Action
Resolving use disparities requires more than physician education. To drive systemic change, hospitals and oncology practices must adopt evidence-based protocols for the use of diagnostics, rather than leave it to the discretion of physicians. Initiating standard protocols such as automatic lab testing or automatic flags in patient records are two potential approaches. Likewise, physicians could be rewarded (or penalized) on the basis of their adherence to protocols. Above all, hospitals and oncology practices—and the health care industry more broadly—must become more transparent about outcomes, and that requires not only a robust tracking system to identify physician performance but also a mandate to take action on the basis of the findings.
Driving systemic change requires a robust tracking system and a mandate to take action on the basis of the findings.
Sometimes progress in health care requires radical new advances in techniques and technology. Other times, it simply requires ensuring that providers and patients follow standard care protocols. By highlighting variations in care among providers and institutions—and the underlying causes of those variations—data and analytics can help generate a clearer picture of how treatments are affecting a given set of patients. In that way, these tools are becoming a powerful new way to help the health care system extend and save patients’ lives.