As regulations, product and portfolio complexity, and costs continue to grow, complaint management is becoming more challenging than ever for biopharmaceutical and medical device companies in the US. The roadblocks are daunting—siloed organizations, intertwined processes, legacy IT systems, and data issues, to name a few. Moreover, the stakes are high. Complaint management gone wrong can result in compliance failures and serious business disruptions.
To succeed in this environment, companies need to adopt a digital-native approach to complaint management, where the entire process is supported by digital technology that augments human capabilities by automating processes and providing real-time visibility and insights. Five components are key: a risk-based approach throughout the complaint management life cycle, streamlined case intake, simplified and consistent processes across business units and products, advanced analytics capabilities to drive novel insights, and an optimized workforce footprint.
Transforming complaint management can improve patient outcomes, revenues, cost savings, and interactions with health authorities.
Transforming complaint management can drive value well beyond compliance. Companies can see improvements in patient outcomes, revenues, cost savings, and interactions with health authorities. Some processes bring accuracy gains of as much as 33% and efficiency gains of up to 30%. Companies thus can absorb the complexity that comes from growth without having to increase resources—and deliver better customer experiences and higher product quality at the same time.
Complaint Management and Its Challenges
Complaint management is the process that health care product manufacturers use to detect, classify, and investigate product complaints (PCs)—an inquiry or statement of dissatisfaction with the identity, strength, quality, or purity of a product. The insights that come from investigations into complaints are used to improve future products.
The end-to-end complaint management process begins with the handling of a complaint and ends with trending, analytics, and providing feedback to R&D and manufacturing to improve product performance. Multiple functions play a role, including product and process design, supply chain and manufacturing, quality operations, quality management systems, and customer-facing functions for patient outreach and education.
The Six Steps of Complaint Management
As shown in Exhibit 1, organizations typically handle complaints in a successive set of processes:
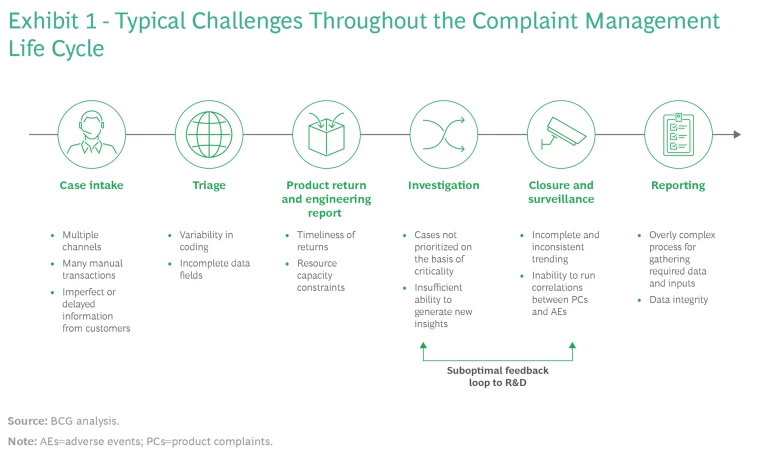
- Case Intake. Receiving and documenting complaints.
- Triage. Prioritizing and processing complaints to determine the severity, reportability, and initial cause of a complaint.
- Product Return and Engineering Report. Identifying the root cause of a problem, usually when a product is returned.
- Investigation. Conducting the appropriate level of investigation and then providing feedback to the relevant upstream function to enable future product improvements.
- Closure and Surveillance. Monitoring complaint information globally and identifying potential trends that require further action.
- Reporting. Notifying regulatory authorities about incidents that meet criteria defined by the US Food and Drug Administration (FDA) and other regulatory bodies.
Each of these steps is riddled with challenges. The case intake process, for example, must contend with multiple channels, a high number of manual transactions, and imperfect or delayed information from customers.
Furthermore, many of the challenges experienced in an initial step can cause delays or other issues downstream. For example, if insufficient data is collected during intake, it may be necessary to collect more information from the patient or health care provider. And even when large amounts of data are collected, it’s difficult to use the information to make better decisions later if the collection processes are not standardized.
The Root Causes of Complaint Management Challenges
The determinants of these challenges are complex. But three root causes stand out above the rest: siloed data and processes, increasing regulatory pressures, and talent issues.
Siloed Data and Processes. If data is siloed in core systems across the enterprise, then access, cross-functional collaboration, and the development of new insights become challenging. This lack of data integration is all too common: our analysis of the top 16 biopharma and medtech companies revealed that, in most instances, data resides in ten or more disparate legacy systems.
These issues are even more challenging for companies with new modalities or combination products, such as:
- Drug and device
- Biologics and device
- Drug, biologics, and device
Most companies’ complaint-handling processes were originally designed to address complaints pertaining to just one type of product. Moreover, the processes were meant to address only complaints and not the adverse events (AEs)—the side effects or safety issues—that combination products can encounter.
Say, for example, that an organization handles PCs and AEs independently for its combination products, with different teams running separate workflows. Consequently, PCs either may not be linked to the AE data for that product, or it could take a while before the data is merged in the workflow. Data is likely to slip through the cracks, especially if the systems aren’t built to integrate the different types of data.
The impacts of siloed data and processes manifest throughout the complaint life cycle, from disparate data sources that are not resolved during the intake process, to inaccurate coding during triage, to missed connections with previous investigations, to incomplete and inconsistent trending during surveillance. All these issues put companies at risk of missing critical information about how their products perform in the market.
To address some of these data challenges, many companies with new modalities or combination products are looking to establish advanced digital capabilities and joint surveillance processes that will handle PCs and AEs concurrently with shared management systems.
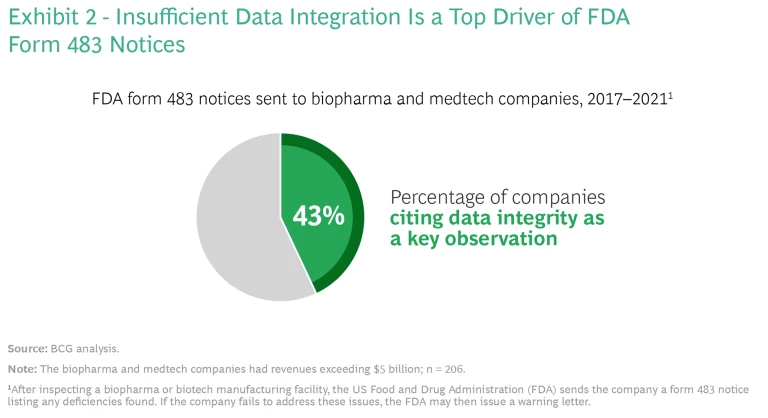
Increasing Regulatory Pressures. Growing regulatory scrutiny has made the need for data integration even more urgent, especially after the implementation of the Sentinel System, an FDA surveillance platform that monitors medical product safety. According to our analysis, data integrity was a key reason for the FDA’s issuance of form 483 notices from 2017 to 2021: 43% of the notices sent to large biopharma and medtech companies (those with revenues exceeding $5 billion) cited data integrity as an issue. (See Exhibit 2.)
Talent Issues. There are simply not enough appropriately skilled people to conduct holistic and sophisticated postmarket complaint handling, investigation, and surveillance. Given the so-called great resignation and the shortage of digital talent overall, the dearth of medically and digitally trained professionals will likely continue to be a challenge for the foreseeable future.
Building a Digital-Native Complaint Management Capability
To succeed in this environment, companies need to build a cutting-edge capability with five key elements. (See Exhibit 3.)
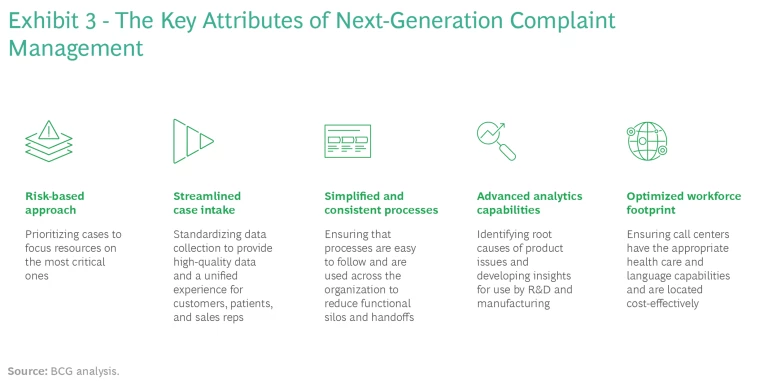
A Risk-Based Approach Throughout the Complaint Life Cycle. Risk-based processes and pathways help prioritize investigations on the basis of the criticality of the risk to patients, giving precedence to the PCs with potentially severe outcomes. A risk-based approach is essential to ensure that sufficient resources are provided for in-depth investigations of those complaints. Here, too, technology and advanced analytics can help develop risk scores on the basis of information supplied in previously filed complaints.
By systematically allocating resources to top-priority complaints, companies can improve the speed and accuracy of such investigations, boosting the overall efficiency of the response. Doing so also helps identify trends and any required corrective and preventive actions much earlier in the complaint life cycle, reducing the risk of penalties for compliance failure.
Streamlined Case Intake. Many intake teams today use multiple channels to collect data, each with its own set of collection processes. As a result, the collected data can be inconsistent. When high-quality data is in limited supply, automated tools, such as machine learning, may not yield high-quality insights.
To avoid this problem, data collection processes must be standardized. Various digital tools can help. For example, natural-language processing can use machine learning to transcribe voice calls to text and identify clusters of themes across complaints to support their classification. And artificial intelligence (AI) can recommend the appropriate cause code for each case.
Companies with streamlined intake processes have a clearer view of data throughout the complaint life cycle. This provides a unified experience for customers, patients, and sales reps; minimizes the need for follow-up calls; and gives investigators the information they need more quickly.
Simplified and Consistent Processes Across Business Units and Products. If a process is very complicated, staff may not follow the proper protocol, which can result in delays and redundant work. This is particularly evident in the investigation process, which is the most time-consuming step in complaint handling. Multiple functions must collaborate closely to analyze relevant data. This often involves sharing spreadsheets and manually observing trends. Without a cross-trained staff who can understand the safety and product quality implications of combination products and new modality delivery systems, significant delays in regulatory reporting and record closures result.
To address these issues, companies need to simplify inordinately complicated processes and ensure that the same processes are followed across the enterprise.
Some companies set up a central dashboard that provides visibility into data from disparate systems, serving as an easy single source of truth for analytics teams and leadership. The dashboard also reduces the need for manual data sharing, freeing up bandwidth for analysis of added value. Algorithms enable faster and more accurate root-cause analysis of trending and excursion (analysis of the number of complaints to see if it is holding steady or exceeding control limits).
Establishing consistent and simplified processes can go a long way toward eliminating functional silos and reducing the number of handoffs between teams.
Advanced Analytics Capabilities to Drive Novel Insights. Problems can arise at any point of the end-to-end process, from manufacturing to the use of the treatment, drug, or device. Strong data analytics capabilities are critical to identify issues and derive the insights needed to address them.
An AI-enabled solution that pulls together disparate data can provide additional capabilities that lead to more insights and can offer powerful support during triage, investigation, and advanced surveillance activities. It can also provide visibility into geographical and other trends, aiding in root cause analysis. At the same time, it can identify causes of complaints and potential correlations to other products with the help of Shapley additive explanation modeling and product manufacturing genealogy tracing.
An Optimized Workforce Footprint. Regardless of where a complaint arises, it must be handled by a global company, with appropriate capabilities (such as the requisite health care background and language fluency) and in a cost-effective manner.
For triage and, in select instances, investigations, companies should set up call centers in locations that can serve multiple geographic regions in varying time zones, not just where the company has customers. But setting up call centers in multiple locations has cost implications. And while labor costs might be lower in some places, finding the right level of talent there—workers who can understand the intent and context behind each complaint—can be a challenge.
A thoughtful footprint for global product evaluation centers can also streamline the returns process, which commonly slows down complaint investigations. This is especially true when cross-border shipments of contaminated devices are involved; compliance with trade and health care regulations can be burdensome.
Significant Impact
Transforming a company’s complaint management capabilities can generate significant impact across functions and stakeholders, including faster response times; lower long-term operational, legal, and regulatory costs; better outcomes; an enhanced customer experience and greater customer satisfaction; and proactive outreach with health authorities. In our work with clients, we’ve seen:
- Efficiency gains of 15% to 30% in PC record processing by automating activities that allow people to better leverage historical information and insights to categorize, consolidate, and investigate records
- Surveillance efficiency increased by 5% to 15% as a result of more effective trending and excursion root cause analysis
- Improvements in accuracy of 33% when using algorithms instead of manual efforts to assign the appropriate cause code
Example 1: A Medtech Company Complaint Management Journey. A US medtech company was having difficulty scaling its complaint-handling capability. Many processes were manual and siloed, which made it difficult to leverage resources effectively. Moreover, every case—no matter how minor—had to be fully investigated, potentially leaving insufficient resources for the cases that were truly serious.
To address these problems, the company designed a new operating model that included the following:
- A standardized intake process with one customer-facing front
- Augmenting triage process with AI tools to classify complaints accurately and efficiently
- A risk-based approach to resource allocation to ensure that the most urgent cases would be prioritized and to reduce the risk of compliance failure
- A standardized evaluation process to reduce the number of handoffs
- Consistent trending and data analytics processes across business units to ensure that one view is provided to regulatory bodies
- Digital enablement to simplify processes and support scalability, consistency, and efficiency
Example 2: A Biopharma Company with Combination Products and Siloed Data. A leading biopharma company that was expanding its portfolio of combination products needed to address the attendant rise in the number of PCs and AEs. The data for conducting postmarket surveillance sat in 15 disconnected systems across the enterprise.
Subscribe to our Health Care Industry E-Alert.
The company launched a series of initiatives to improve its complaint-handling capabilities. It redesigned the structure of its complaint management organization and defined clear roles and KPIs to establish clear points of accountability and minimize handoffs. The company also created a streamlined cross-functional joint surveillance and investigation process for all PCs and AEs stemming from the same combination product. In addition, the company embedded AI and other modern technologies in the business process to enhance data management and analytical insights. These pragmatic initiatives brought immediate improvements, and the cost savings they generated were enough to cover the initial digital investments.
Getting Started
How should companies determine whether their complaint management process is sufficient for their modalities and products? We recommend starting with three actions.
Assess your current complaint management processes. First, a company needs to evaluate its portfolio holistically for risks. Is the complexity of the product portfolio growing? Is the workforce footprint becoming more geographically complex? Have the timelines for closing on complaints become lengthier? A mock audit will reveal if the company understands the impact of any event on the product across the supply chain. For example, if the temperature of the shipment exceeded the recommended range while the product was in transit, did the company test the product to determine whether it had been damaged?
The next step is to evaluate capabilities throughout the life cycle of the complaint. How long does it take to achieve resolution? Are the costs increasing? Where are the bottlenecks? Looking at leading indicators or conducting a simulation should reveal whether there’s a problem and what the transformation priorities should be.
Make sure your people and processes support your objectives. Companies need to make sure that their complaint management organization is set up correctly. This means determining where the complaint management process lives—which function is responsible and which capabilities are needed to support the various processes.
Organizations also need to start thinking about how they can set up streamlined and consistent risk-based processes across their complaint-handling organization. It’s important to apply safeguards where they can have meaningful impact on product quality, safety, and patient experience.
In addition, companies should make sure that they have the requisite talent—people with the deep analytics skills required for holistic and sophisticated postmarket investigation and surveillance. To enable continuous improvement and increase accountability for outcomes at the mid- to upper-management levels, staff must be able to track key efficiency and reliability metrics.
Get data and tools in working order. Given the critically important role that data and analytics play, companies need to make sure that their data and technologies are in working order. Rather than rush to get the latest tools, companies should focus on how to use technology smartly to improve human decision making, processing speed, and efficiency.








