Amid the quick uptake of generative AI (GenAI) in many industries, most medtech companies have been hesitant about implementing it. But waiting too long risks putting these companies at a permanent disadvantage in adopting this fast-moving technology. According to a global BCG study on the maturity and value of GenAI, 24% of companies across sectors have created value from the technology. In contrast, only 10% of medtech companies have created value from GenAI, with 90% seeing limited or no measurable impact. Even within health care, sectors like biopharma are already applying GenAI to create a broad range of documents—in areas such as R&D, manufacturing, and sales and marketing. Medtech can follow in the same path, particularly for internally facing processes like regulatory filings and quality management.
Regulatory and quality processes are a major bottleneck for medtech companies; they entail the creation and refining of many complex documents, making them important potential applications for GenAI in medtech. We have identified use cases where medtech companies can apply existing GenAI solutions in regulatory and quality processes and make dramatic gains in efficiency, accuracy, and other key metrics. As with any application of AI technology, GenAI in medtech requires a layer of human oversight and governance. But with a structured approach, heads of regulatory and quality functions at medtech companies can follow the lead of other health care segments and begin capitalizing on the technology—starting today.
The Evolution of Medtech Regulation
The regulatory situation for medtech players is changing. The EU’s Medical Device Regulation (MDR); cyber and patient data protection laws; increased requirements for clinical evidence; more formalized regulations for digital health or software-as-a-medical-device solutions; and changes to in vitro diagnostics (IVD) regulations have made the process of developing and selling medical devices and services far more costly and complex.
A shortage of skilled regulatory labor exacerbates the challenge. In some cases—for example, during the transition from the previous EU Medical Device Directive to the MDR—some companies have been unable to get products through regulatory certifications or had to pull existing products from the market as a result, with significant implications to their business.
Even for approved products, companies face challenges in managing the interdependency among highly complex regulated documents, requiring meticulous coordination to ensure consistency across all related documents at all stages of a product’s lifecycle.
Benefits of GenAI in Managing Quality and Regulatory Requirements
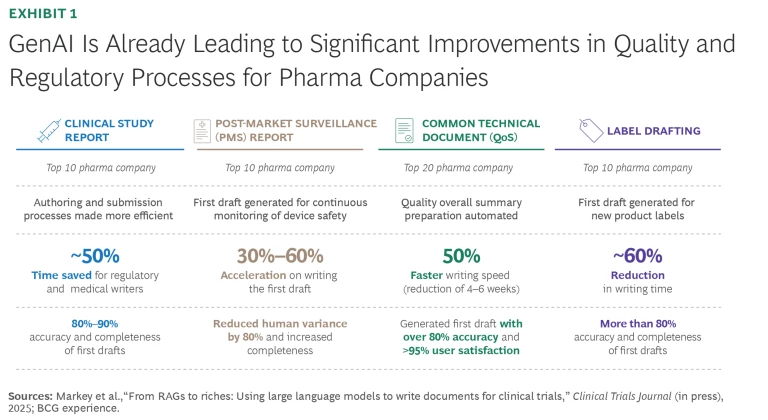
Within this environment, GenAI can lessen the workload for quality and regulated documentation processes. In fact, early adopters in health care are using traditional and generative AI to innovate, automate, harmonize, and simplify elements of their quality management systems and regulatory affairs functions, gaining a real competitive advantage. In our work with a handful of vanguard biopharma clients, we have seen measurable gains in performance. (See Exhibit 1.)
Specifically, GenAI solutions can perform the following tasks.
- Interpret, review, and summarize text, audio, or visual data, such as reviewing test data or user feedback.
- Generate first drafts of quality and regulatory documentation for further refinement and human review. At a top 10 biopharma client, GenAI has led to reductions in writing times of up to 60% for regulatory labeling use cases, 70% for clinical trial protocols, and 70% in clinical study reports.
- Manage interdependencies, ensuring consistency and accuracy across all related documents during a device’s lifecycle when product updates or updates of the regulatory environment trigger changes in technical filings. An organization accelerated the process of scoping an initial draft by 50% to 70% and improved cycle times for a regulatory dossier by four to six weeks.
- Enable collaboration and accelerate reviews by consolidating input from cross-functional teams. More broadly, the ease of use and high accuracy in eliminating potentially burdensome tasks leads to a better employee experience, including user satisfaction ratings of more than 95%, and accuracy of over 85% at a large biopharma company.
The Most Promising Use Cases for Medtech
Applying GenAI to regulated document generation and regulatory and quality management processes (given their complexities and broad dependencies) holds significant untapped potential, and companies can apply technical approaches to mitigate risks. We have identified more than 10 high-value use cases within both regulatory and quality processes. In this article, we focus on high-priority cases that are easiest to deploy and offer the greatest potential value for medtech, based on our biopharma experience.
Use Cases for Regulatory Documentation and Processes
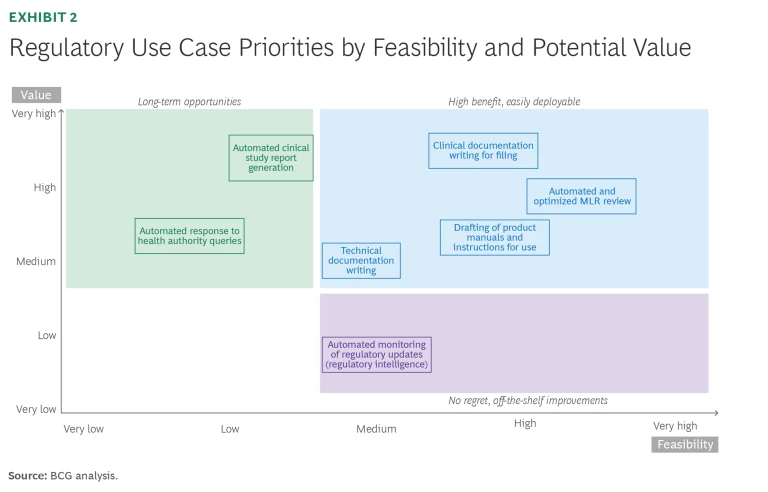
Technical documentation writing for regulatory filings. GenAI can potentially help generate technical documents in the medtech industry by automating content creation, which can improve efficiency while also enabling quick and easy human-in-the-loop reviews to confirm regulatory compliance. GenAI could automatically generate first drafts for various technical and clinical documents for medtech companies too—such as product specifications, design history files, risk assessments, and validation protocols—by leveraging predefined templates and historical data. The data can help add advanced analytics, using AI on top of the GenAI capabilities. GenAI tools can be provided with context on current regulatory standards, such as those from the Food and Drug Administration, International Organization for Standardization (ISO), and EU MDR guidelines, ensuring that generated documents align with requirements. GenAI to support writing could reduce time spent on initial drafting by 40% to 60% and ensures a consistent structure and format across documents irrespective of the writer.
Automated medical, legal, and regulatory (MLR) reviews and adjacent processes.
GenAI can also significantly enhance the MLR review process by streamlining workflows for regulatory teams. For medical review, it can analyze clinical data and references to assess the compliance of any claims based on that data to generate the claims libraries. For regulatory, it can cross-check any promotional material across these references and claims libraries. For legal reviews, GenAI checks promotional materials against regulatory standards and assesses existing patents to identify IP risks. Across the board, GenAI can make recommendations on changes for MLR and refine text directly.
Product manual drafting (labeling). GenAI can significantly enhance the drafting process for product manuals and instructions for use (IFUs) by automating content creation, ensuring regulatory compliance, and streamlining the documentation workflow. GenAI can create initial, well-structured drafts and support post-approval changes during the product lifecycle. It does this by extracting information from technical specifications, design documents, and user requirements while maintaining compliance with required regulatory standards. GenAI can also adapt the language to make complex technical information understandable for a non-specialist audience, including patients and caregivers, with guidance for suitable reading age. For medtech products distributed internationally, GenAI can also automatically translate product manuals and IFUs into multiple languages while maintaining the original content’s meaning, enabling faster assessment that the translation was effective.
Use Cases for Quality Management Documentation and Processes
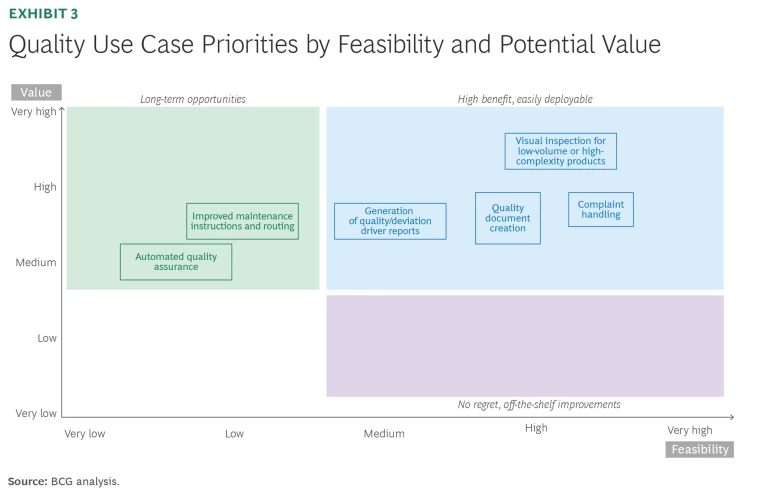
Complaint handling. GenAI-enabled tools can streamline how complaints are managed, investigated, and resolved. Tools can automate the intake and classification process for incoming complaints by analyzing the content and identifying keywords, patterns, and severity levels. GenAI can cross-reference complaint data with other quality records, such as past deviations, manufacturing data, and maintenance logs, to efficiently make recommendations on potential root causes (while companies control for potential bias). The technology can also generate corrective and preventive actions (CAPAs), draft complaint reports, and proactively identify emerging risks in products or processes based on ingested information for further validation by a human in the loop.
Quality document creation. GenAI can generate initial drafts of common quality management documents—such as standard operating procedures, product specifications, and validation protocols—by drawing from existing templates, regulatory guidelines, and historical data. The tools could automatically incorporate required language and eventually maintain strict version control for traceability. (This functionality is still in development for many GenAI solutions.) Quality documents often require input from multiple departments—such as R&D, manufacturing, and quality assurance—and data sources. GenAI can holistically pull relevant data from various systems, such as customer feedback, product test results, manufacturing logs, and field performance data, thus improving document accuracy and completeness.
Deviation handling, including deviation driver reports. Generative AI can transform the entire deviation investigation and management process for medtech companies. It features tools that clarify trends, classify deviations by severity, identify root causes, and recommend appropriate corrective actions. As before, these would need to be assessed by a human in the loop to make sure that the GenAI is not introducing bias and/or the historical information was incomplete and therefore generating bias.
Which Solutions Are Currently Available?
Based on our experience, there are
three distinct approaches
for implementing GenAI, with different ambition levels.
- Deploy. The most straightforward option is to leverage configurable, off-the-shelf GenAI tools that can make routine tasks such as translations or knowledge management more efficient. These solutions are already widely available and can be configured to the needs of medtech companies.
- Reshape. The next level of ambition is to transform entire functions through GenAI to create new ways of working and different processes, leading to greater benefits by building on existing GenAI solutions available off-the-shelf or built by other players.
- Invent. The highest level of ambition is to create new GenAI-powered solutions. Given that some health care companies are generating high-quality output without the need to build proprietary large language models (LLMs), we don’t expect this will be relevant for medtech companies.
Within the first two categories—deploy and reshape—several companies from startups to larger players have launched products for AI- and structured-content-authoring-supported regulatory and quality tasks, including GenAI. For regulatory processes, the first commercially available offerings focus on regulatory filing support, drafting of regulated documents including trial reports or product manuals, or monitoring of regulatory updates. The industry is maturing quickly, with providers adding features and tools all the time, although in most cases biopharma companies are finding that they need to customize heavily and/or build internal capabilities to scale across multiple use cases. Although medtech companies can start with foundational off-the-shelf offerings, in regulatory and quality there is a need to customize tools and layer on additional capabilities to ensure the right level of quality.
For quality management, traditional electronic systems are increasingly augmented by AI and GenAI functionalities. These include predictive analyses, auto-categorization and insight generation, or GenAI chatbots to extract quality program data.
In the second category—reshape—companies mostly customize existing platforms that integrate GenAI holistically into end-to-end processes, leading to sizable gains in efficiency and effectiveness across business functions. For example, BCG has developed a proprietary tool (Editor AI) using existing LLMs to write regulatory and quality documents. The tool generates full first drafts of many document types and can orchestrate the drafting of interdependent sets of documents, right through to submission. Besides providing the software, BCG also supports companies in adapting their processes to the new ways of working—which our experience has found to be critical in generating value, as otherwise the drafting time is reduced but other process roadblocks appear. As a result, companies can reduce their manual workload for some steps, such as drafting, by up to 70%, produce outputs faster, and improve their accuracy and documentation quality at the same time. (A recent study found that using a tool to prompt LLMs with accurate, up-to-date information dramatically improved the quality of GenAI-created drafts of documents for clinical trials.)
Subscribe to our Health Care Industry E-Alert.
Getting Started with GenAI in Medtech
The vanguard of early adopters in health care has already generated a set of best practices that medtech companies can apply to move past the hype in GenAI and begin implementing it for regulatory and quality processes.
Start small and think big.
Define a clear, end-to-end transformation strategy focusing on regulatory and quality management topics. Begin with small-scale projects specifically targeting one or two key processes—prioritized by feasibility and impact—to quickly show value (in terms of speed, time savings, quality improvements, and headcount reductions), gain practical knowledge, establish foundational capabilities, and build momentum for larger-scale change. Quick wins to keep the organization engaged and show value along the way are key.
Balance automation with human oversight.
Implement stringent quality-assurance and transparency mechanisms within GenAI tools for regulatory use, as they often would be classified as higher risk by the EU AI Act and regulators. Ensure that a human remains in the loop to maintain oversight, mitigate risks, and uphold regulatory and quality compliance at all times. As the technology evolves, there are plans to validate these tools in line with EU AI Act and FDA requirements for digital systems so that traceability, versioning, bias, reproducibility, and specificity can be confirmed. Tools are also available to challenge the robustness of GenAI solutions by creating different scenarios to assess in terms of accuracy.
Build human-centered design into solutions.
Test the system with users to create change champions and super users who can advocate for the tool and its design after deployment. These people can also provide valuable quality control and functionality reviews as you customize GenAI tools. Otherwise, we have seen adoption drop if the change is seen as purely technology-driven in terms of workflows and functionality.
Establish a positive narrative and get employee buy-in.
Clearly communicate the regulatory and quality benefits of GenAI in your narrative, focusing on what the change will mean for individual users. Provide comprehensive training focused on regulatory compliance and quality assurance for key users, so they know that critical processes will not be compromised but potentially enhanced with GenAI, with a human in the loop at all times. Establish new roles and ways of working to support GenAI processes in regulatory and quality contexts to enable a true transformation.
GenAI is transforming a broad range of industries as well as other health care segments, including biopharma. Most medtech companies haven’t capitalized on the technology just yet, but they can start to close the gap by applying it to regulatory and quality management where biopharma has already seen early successes. In doing so, they can make internal processes far more efficient and accurate, create a better employee experience, free up capacity for higher-value tasks, and build the critical capabilities they will need for future GenAI applications.
The authors would like to thank Laurence Birdsey, Alan Grimm, and Sören Grothkopp for their contributions to this publication.








